Novel Direct Arylation and Amination Reactions: Rapid Synthesis of Functionalized Biaryls, α-Arylated Ketones, Arylamines and Heterocycles
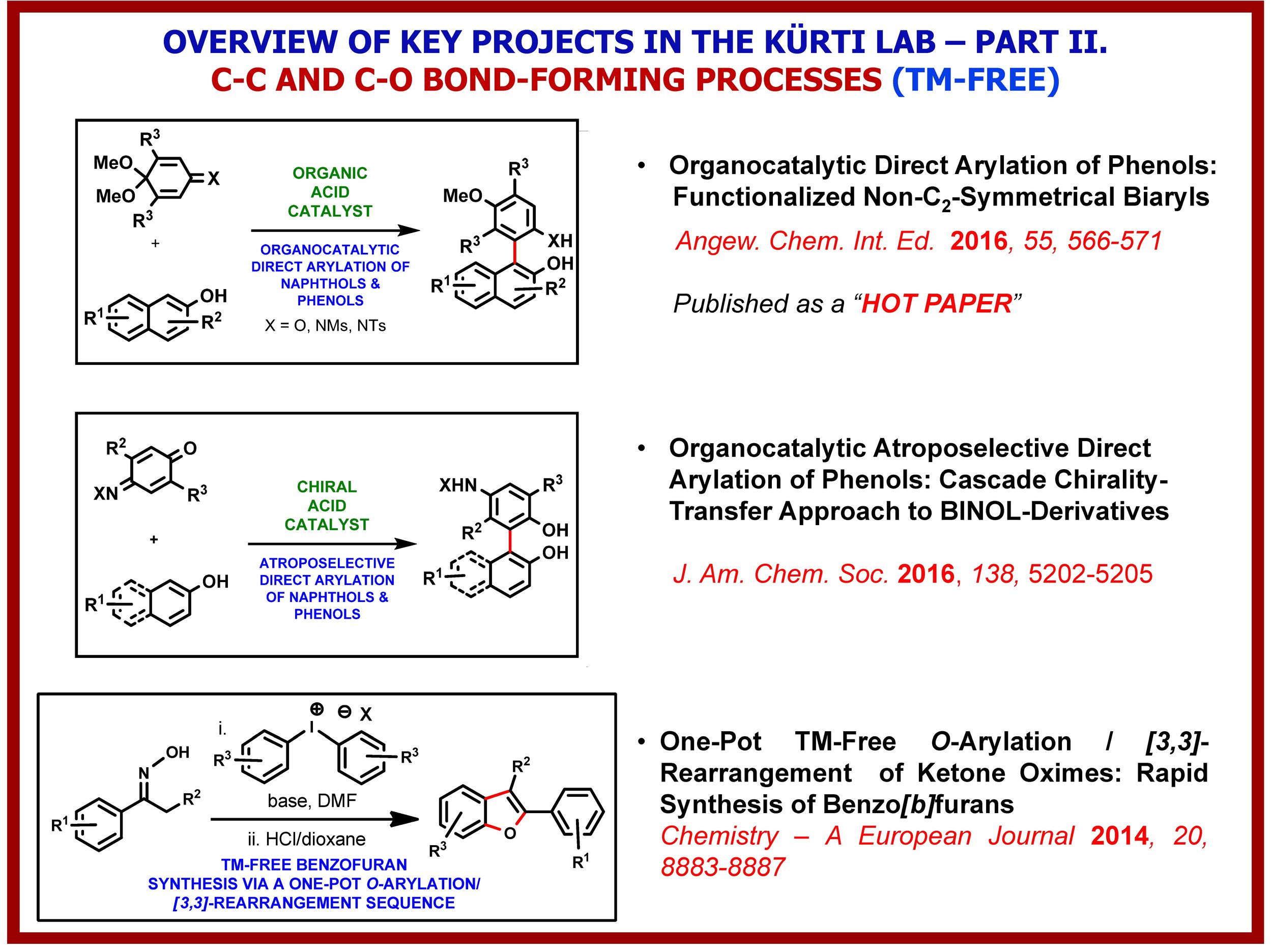
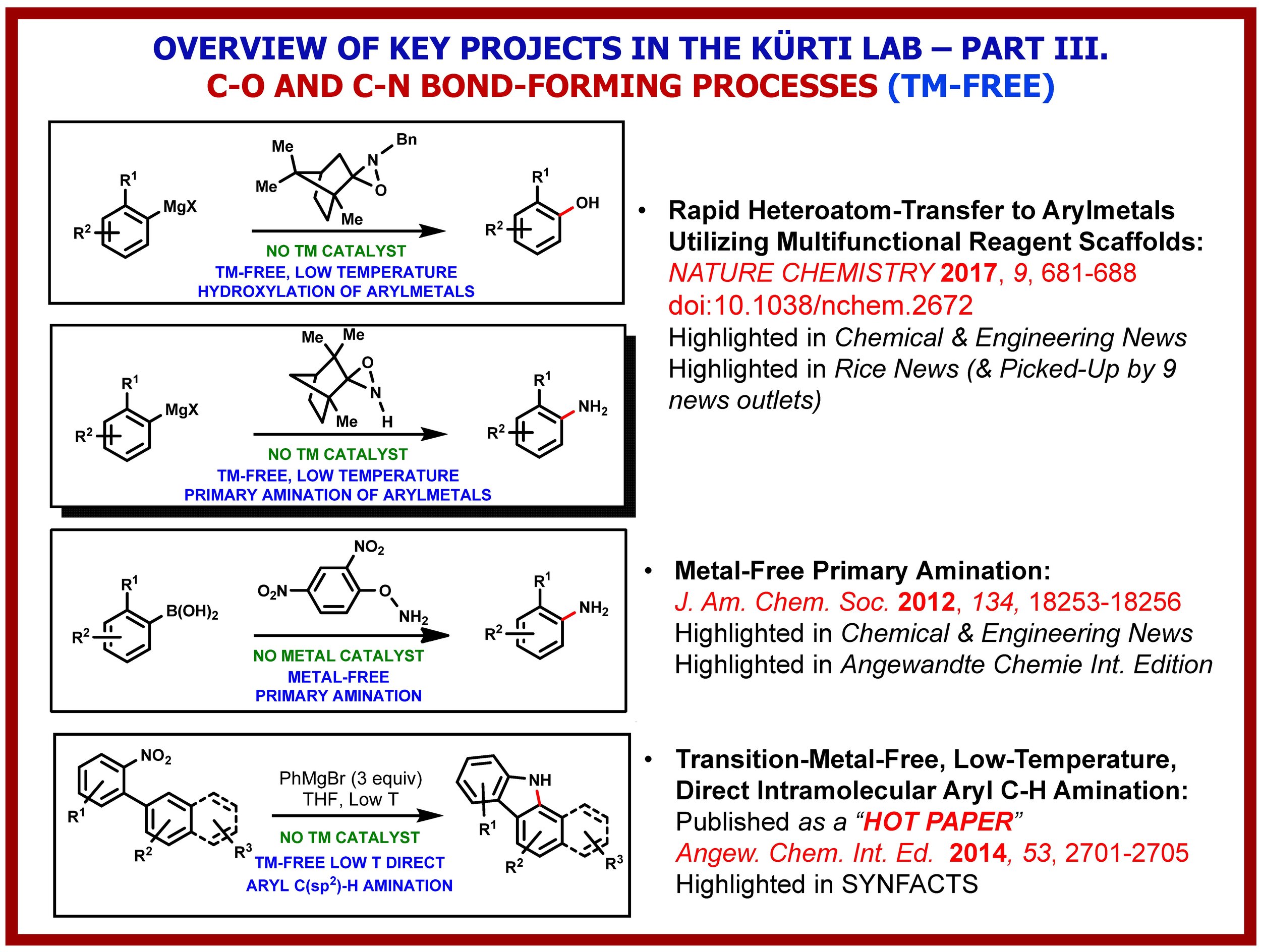
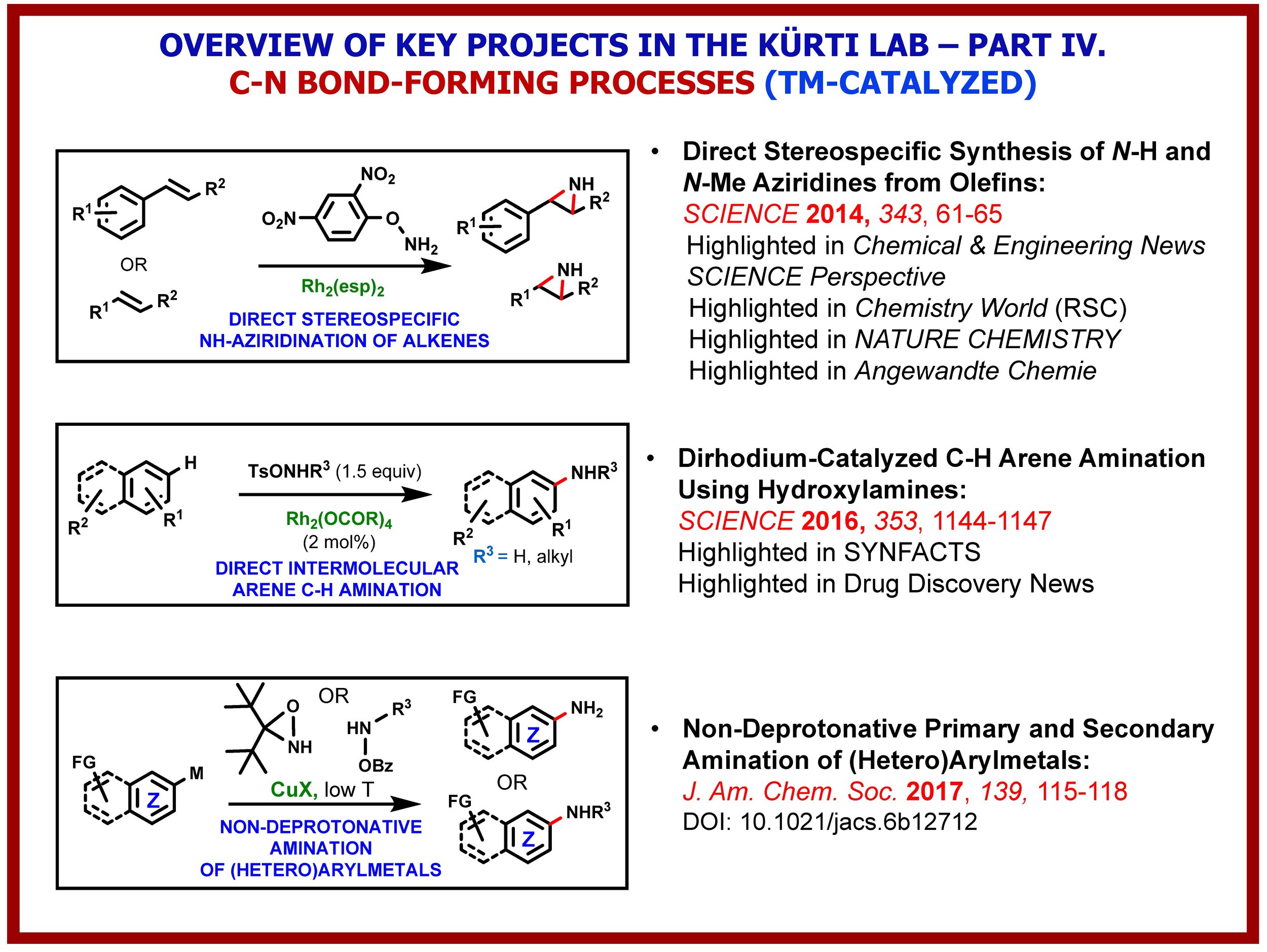
The growth of knowledge in the chemical sciences has seen a dramatic increase since the beginning of the 21st century. The advance is particularly significant in synthetic chemistry because of its centrality and value to society. Key to all of this is the constant development of novel C–C and C-heteroatom bond-forming strategies, methods and reagents that expand the toolbox of synthetic organic chemists and enable the environmentally friendly construction of complex molecular structures using the fewest number of chemical steps and generating the least amount waste. The Kürti lab has been exploring several fundamentally new strategies for the transition-metal-free direct: (i) arylation of arenes; (ii); a-arylation of ketones, esters and amides; (iii) O-arylation of oximes; (iv) primary amination of arylboronic acids and (v) intramolecular C(sp2)–H amination of arenes. We have also discovered, in collaboration with the Falck (UTSW) and Ess labs (BYU), the Rh-catalyzed direct N–H/N–alkyl aziridination of olefins, a transformation that eluded synthetic chemists for decades. These methods have one common feature: a weak N–N or N–O bond is cleaved in order to form a stronger C–C or C–N bond. In-depth experimental and computational studies have already identified the critical factors required for efficient alkyl-aryl, aryl-aryl, O-aryl and N-aryl bond-formation and led to several innovative and environmentally benign methods for the rapid preparation of structurally diverse arylated carbonyl compounds, functionalized biaryls as well as O- and N-heterocycles.